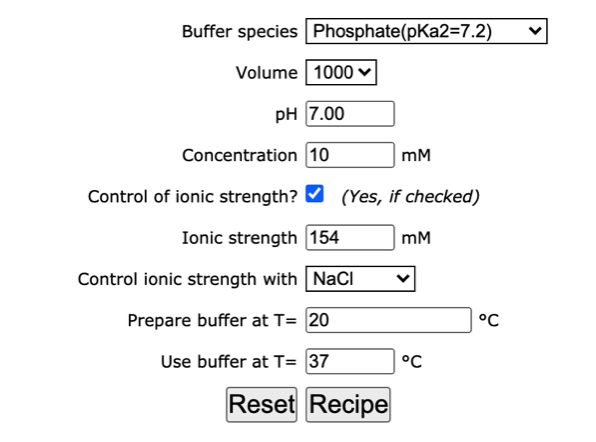
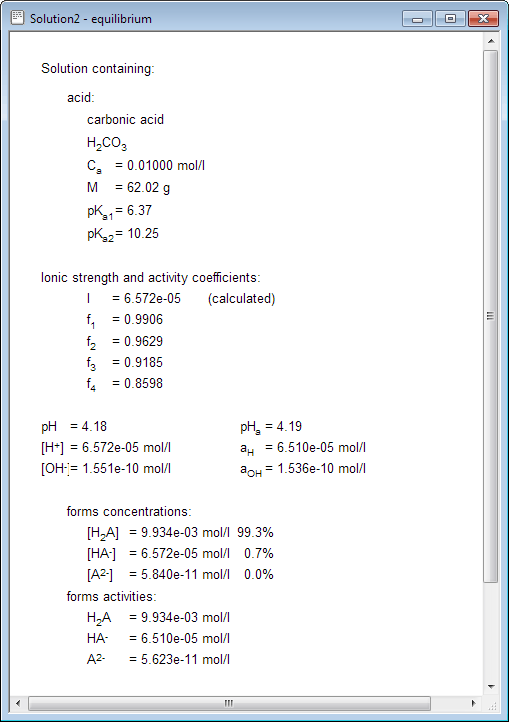
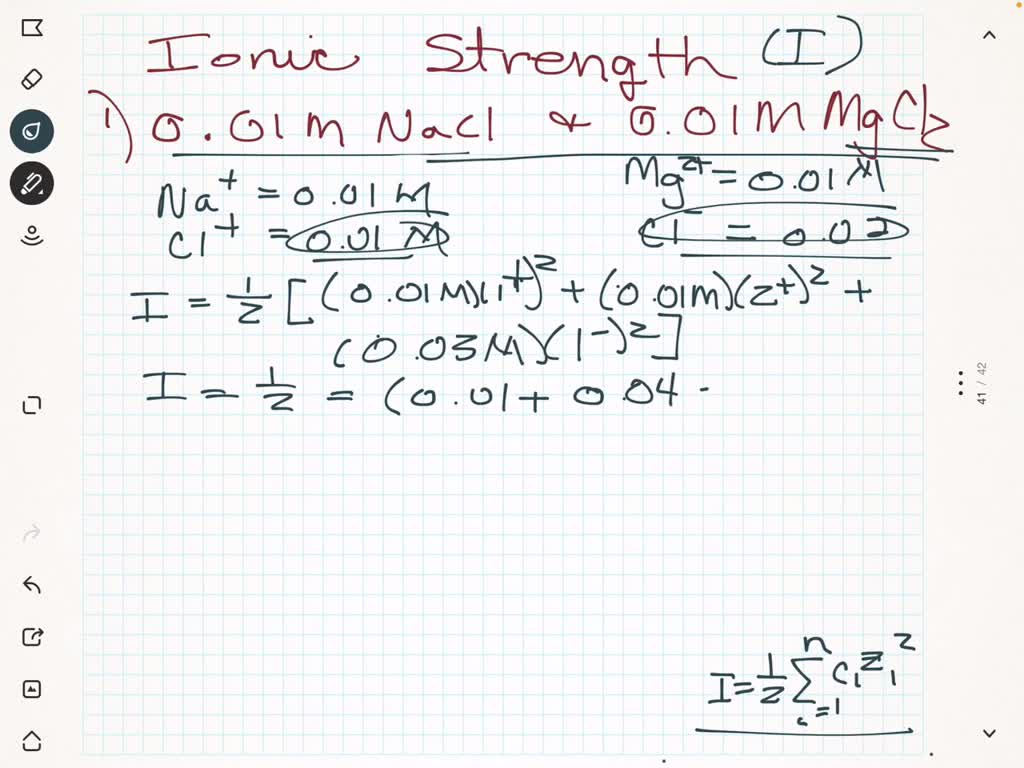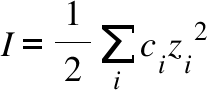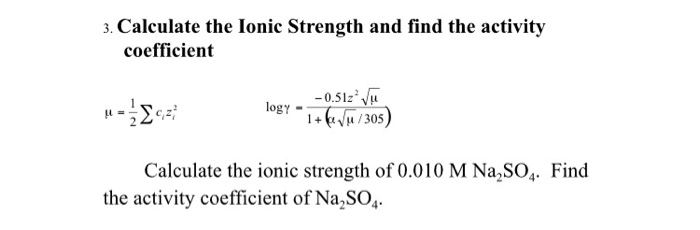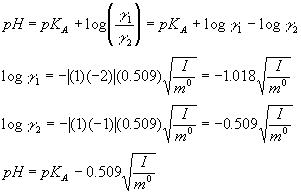CHAPTER 5 ELECTROLYTE EFFECTS AND EQUILIBRIUM: CALCULATIONS IN COMPLEX SYSTEMS Introduction to Analytical Chemistry ppt download

Calculation of Ionic strength||How to calculate ionic strength ||Rank booster-3||Cpet-2021|| - YouTube

Example Write a charge balance equation for a solution containing KI and AlI3. Solution KI g K+ + I- AlI3 = Al I- H2O D H+ + OH- The equation can. -

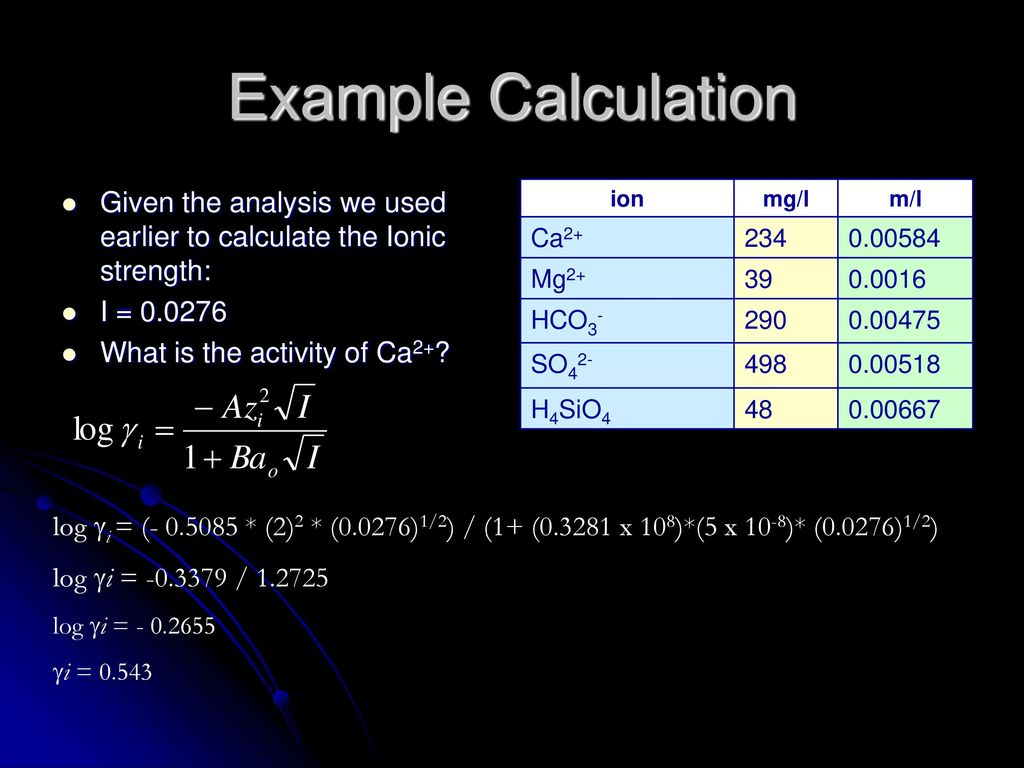
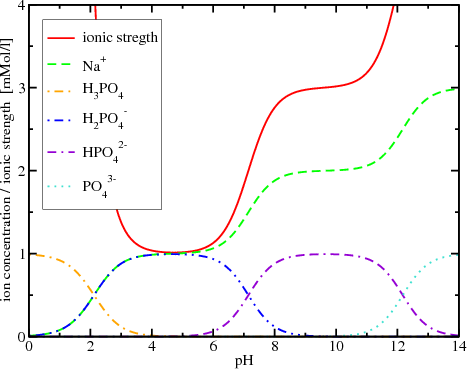
pH calculations and more in fundamentals of pharmaceutics. : What is ionic strength of solutions and how is it calculated?

Example Write a charge balance equation for a solution containing KI and AlI3. Solution KI g K+ + I- AlI3 = Al I- H2O D H+ + OH- The equation can. -
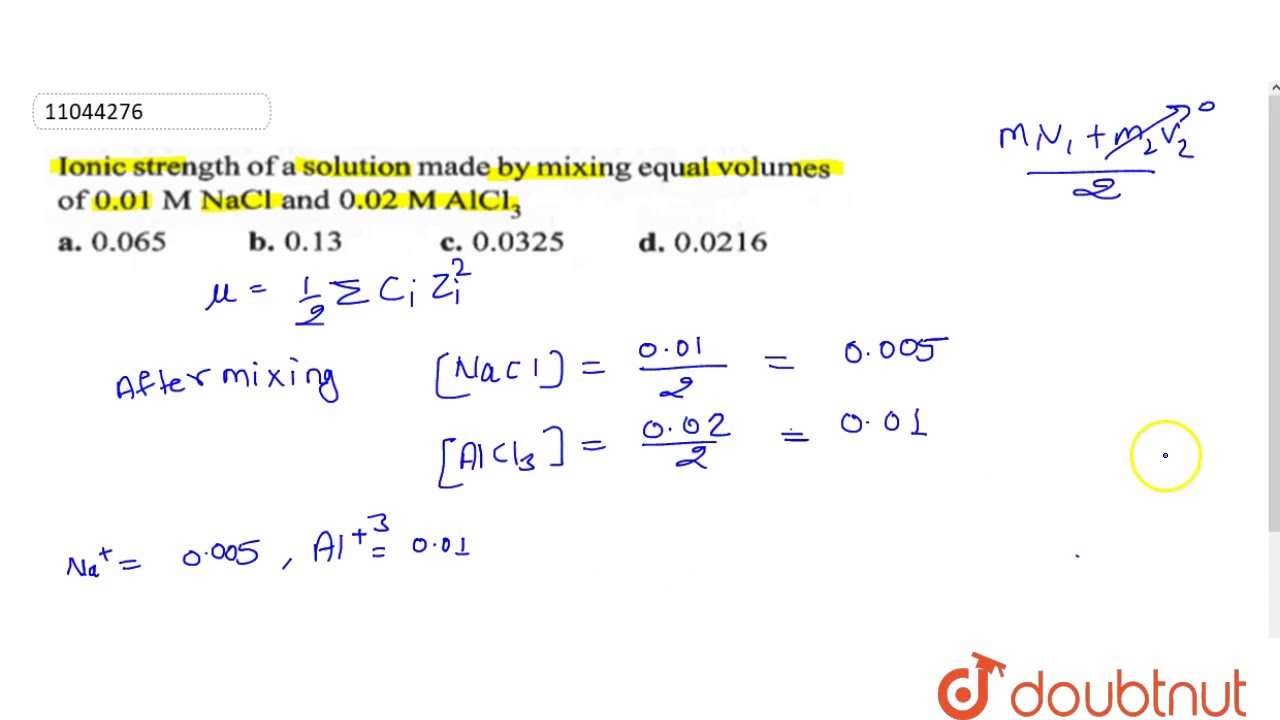
Ionic strength of a solution made by mixing equal volumes of `0.01 M NaCl` and `0.02 M AlCl_(3)` - YouTube
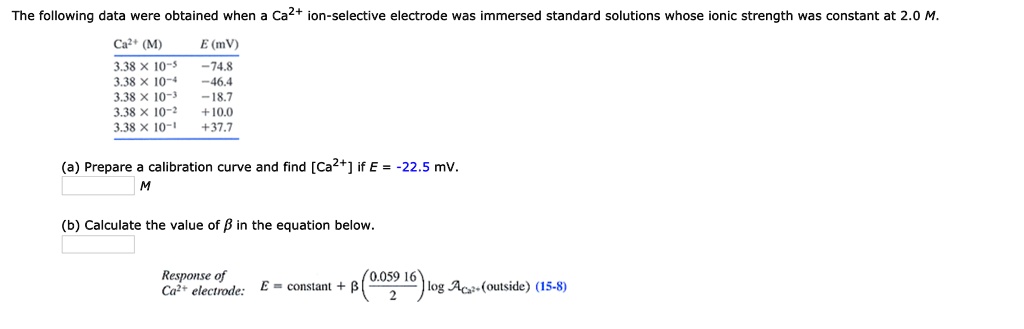
SOLVED: The following data were obtained when Ca2+ ion-selective electrode was immersed standard solutions whose ionic strength was constant at 2.0 M. Cat (M) E (mV) 74.8 46.4 18.7 I0.O +37.7 3,38 *