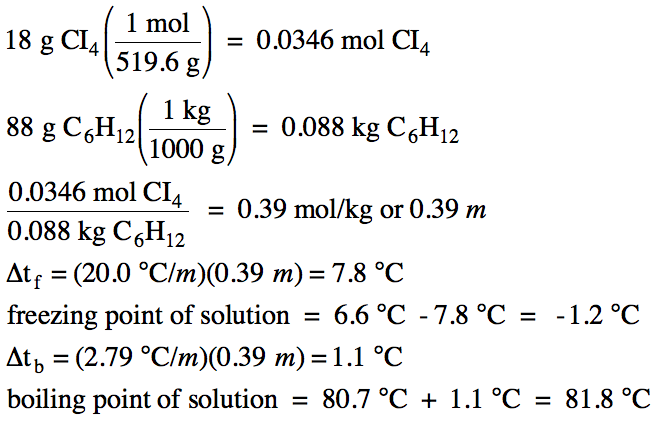
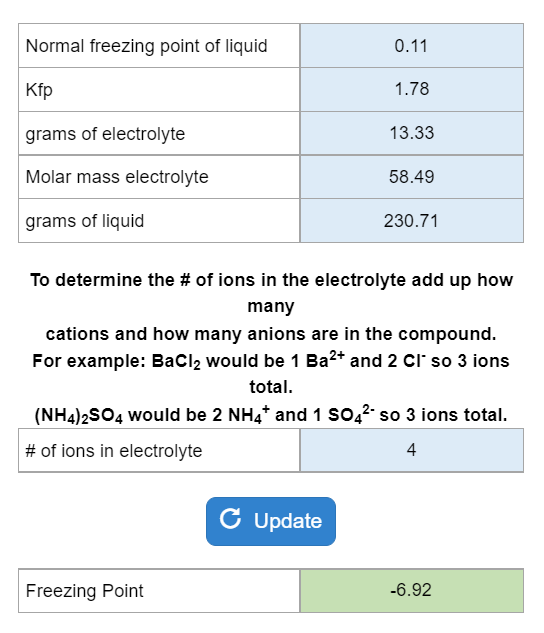
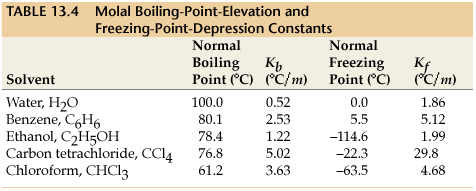
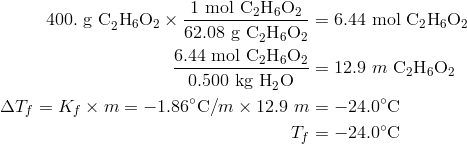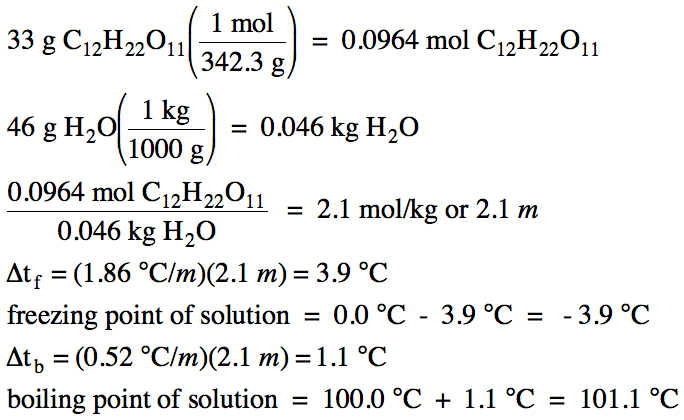calculate molal depression cons†an t of solvent , which has freezing point 16.6^0 celsius, andblatent heat of fusion 180.7
![Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ] Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ]](https://i.ytimg.com/vi/zGfIbhioFZ0/maxresdefault.jpg)
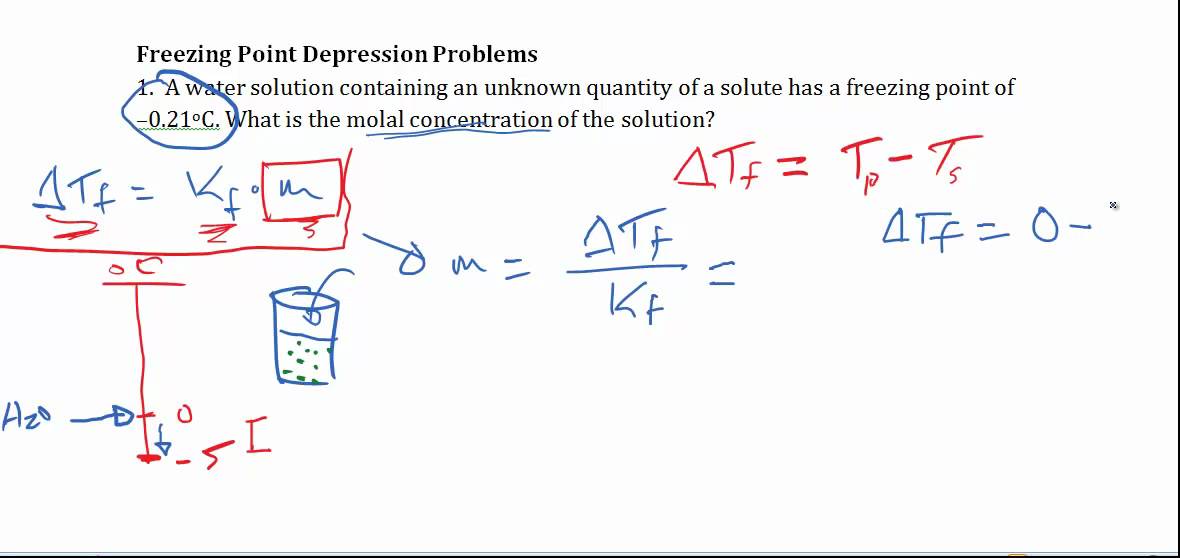
Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. [ Ka = 1.4 × 10^-3, Kf = 1.86 K kg mol^-1 ]
Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180 g mol^-1) in 250 g of water. - Sarthaks eConnect | Largest Online Education Community

How do you find the freezing point of pure water from the freezing point depression equation? | Homework.Study.com

Calculate the freezing point and the boiling point at 1 atmosphere of a solution containing 30 g cane sugar (molecular mass 342 ) and 150 g water.Given : Kb = 0.513 and Kf = 1.86