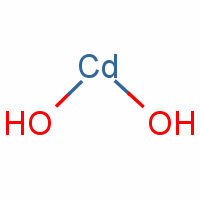
Effects of laser fluence on the Cd(OH)2/CdO nanostructures produced by pulsed laser ablation method | SpringerLink
The molar solubility of Cd(OH)2 is 1.84 × 10^–5 M in water. The expected solubility of Cd(OH)2 in a buffer solution of pH = 12 is : - Sarthaks eConnect | Largest Online Education Community

Synthesis of cadmium hydroxide nanostructure via composite-hydroxide-mediated approach - J Adnan, M Arfan, T Shahid, MZ Khan, R Masab, AH Ramish, S Ahtasham, AG Wattoo, M Hashim, A Zahoor, MF Nasir, 2019

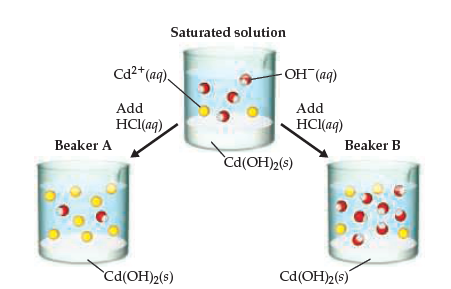
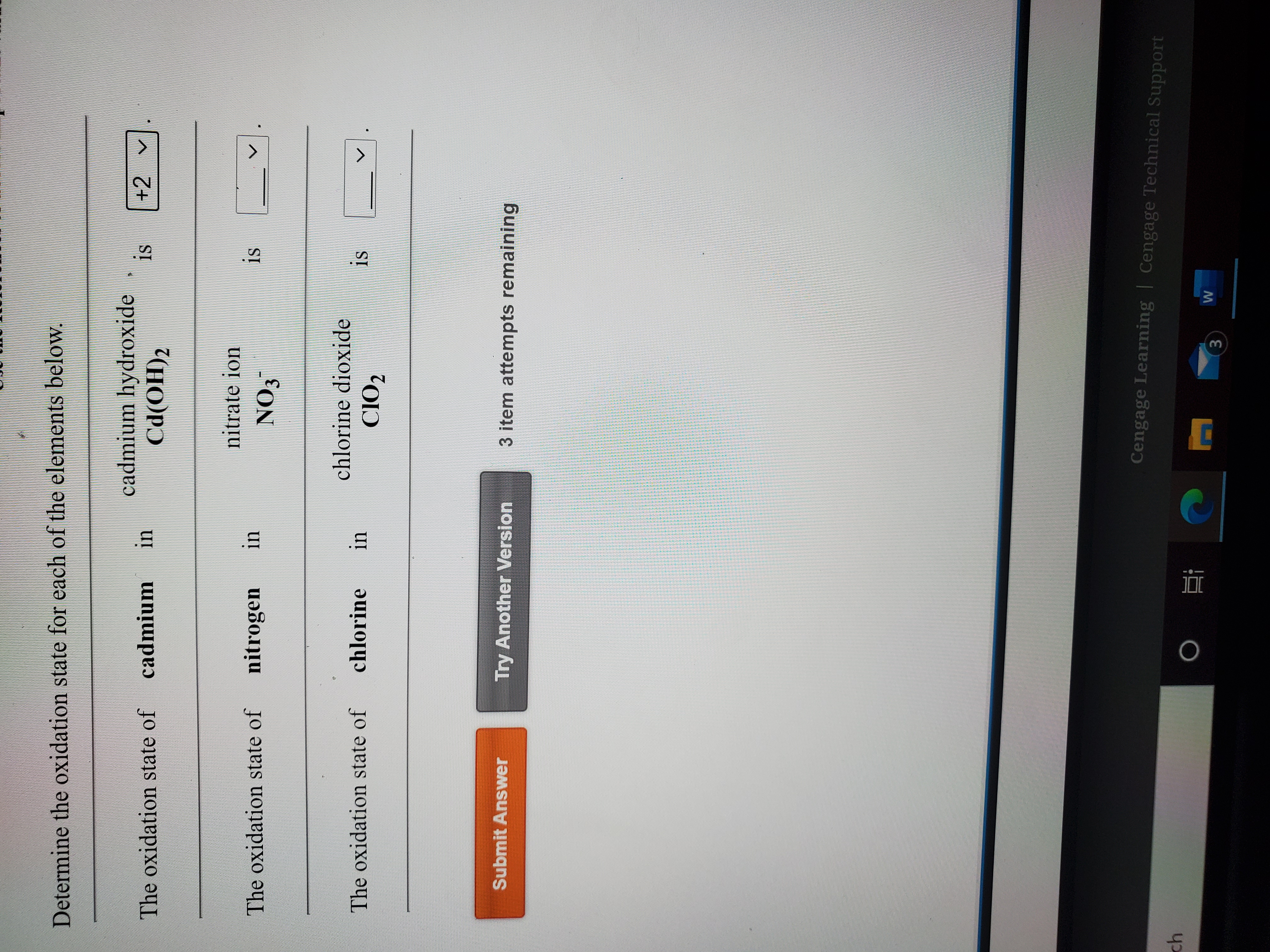
Given the cell: Cd(s)|Cd(OH)2(s)|NaOH(aq, 0.01 M)|H2(g, 1 bar)|Pt(s) with Ecell = 0.0 V . If E^oCd^2 + |Cd = - 0.39 V , then Ksp of Cd(OH)2 is:

Effects of laser fluence on the Cd(OH)2/CdO nanostructures produced by pulsed laser ablation method | SpringerLink
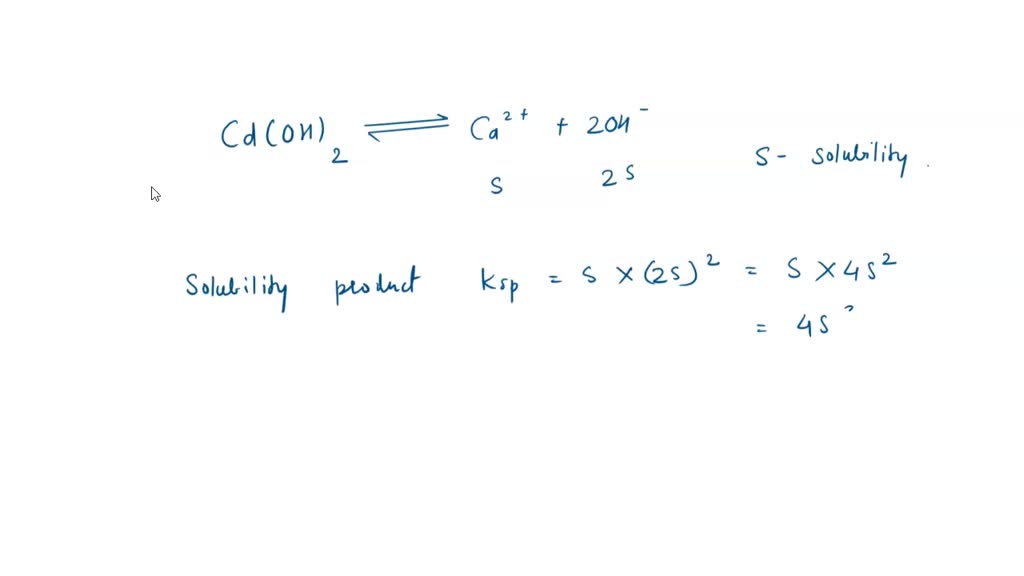
SOLVED: The solubility product constant for Cd(OH)2 is Ksp = 2.5 × 10−14 at 25 °C. a. What is the molar solubility of Cd(OH)2? b. What is the mass solubility of Cd(OH)2

Synthesis of cadmium hydroxide nanostructure via composite-hydroxide-mediated approach - J Adnan, M Arfan, T Shahid, MZ Khan, R Masab, AH Ramish, S Ahtasham, AG Wattoo, M Hashim, A Zahoor, MF Nasir, 2019