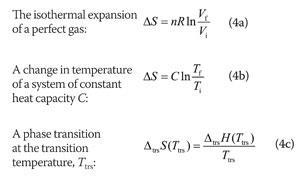
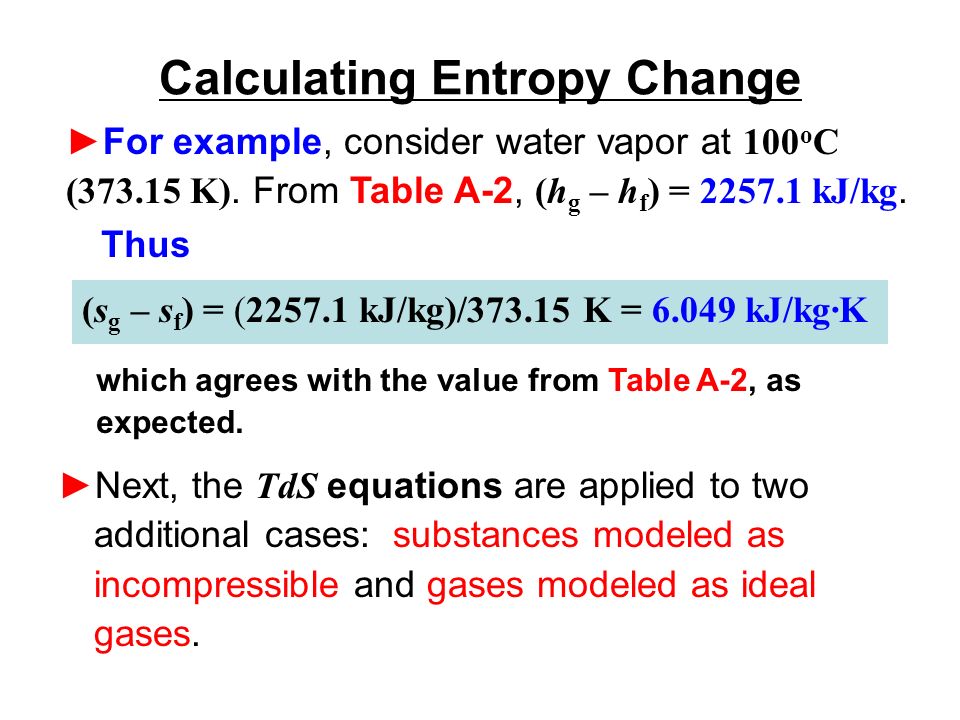
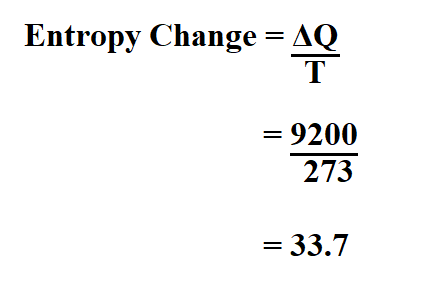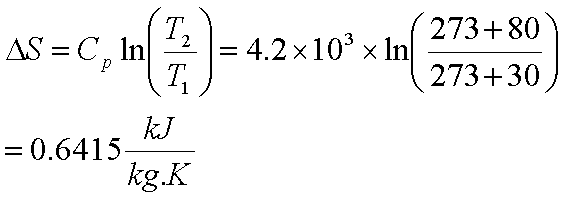Entropy Change For Melting Ice, Heating Water, Mixtures & Carnot Cycle of Heat Engines - Physics - YouTube

Calculate the entropy change in surroundings when `1.00` mol of `H_(2)O(l)` is formed under stan... - YouTube

How to Calculate the Entropy Change for a Chemical or Physical Process Based on Absolute Entropies | Chemistry | Study.com

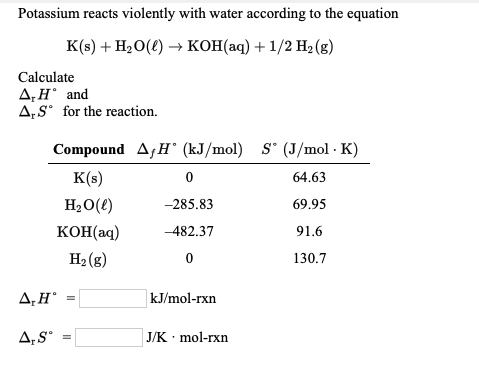
Calculate the entropy change in surrounding when 1.00 mol of H2O(l) is formed under standard condition fH^ = - 286 KJ mol^-1 .

Calculate the entropy change in surroundings when 1.00 mol of H2O (l) is formed under standard conditions at 298k, Given ,H^o = - 286kJ mol^-1

![15.2/R1.4 Calculate the standard entropy change for a reaction [HL IB Chemistry] - YouTube 15.2/R1.4 Calculate the standard entropy change for a reaction [HL IB Chemistry] - YouTube](https://i.ytimg.com/vi/IwRy4iYVQLI/maxresdefault.jpg)