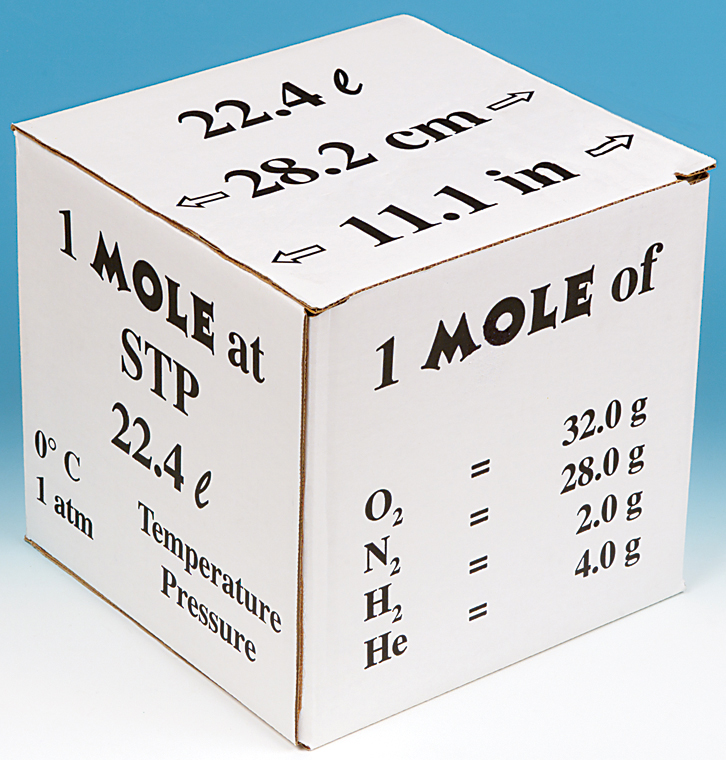
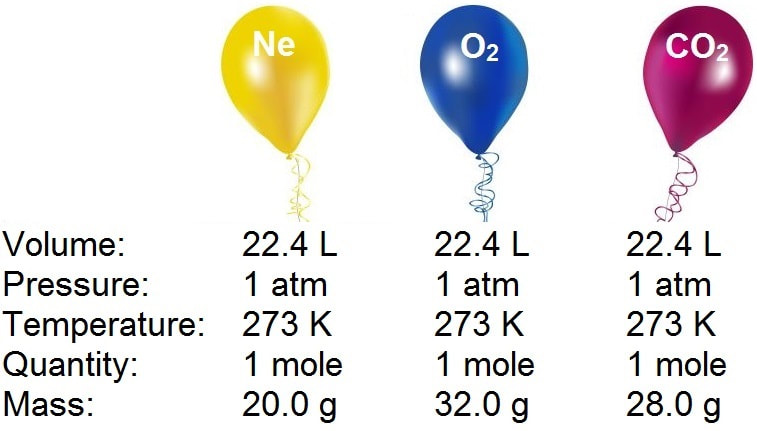
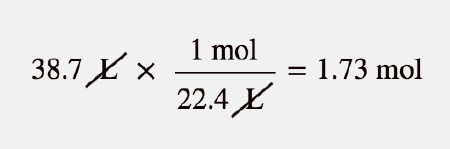When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2(g) , each at STP, the moles of HCl(g) formed is equal to :

When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2(g) , each at STP, the moles of HCl(g) formed is equal to :

When 22.4 L of H(2)(g) is mixed with 11.2 of Cl(2)(g), each at STP, the moles of HCl(g) formed is equal to

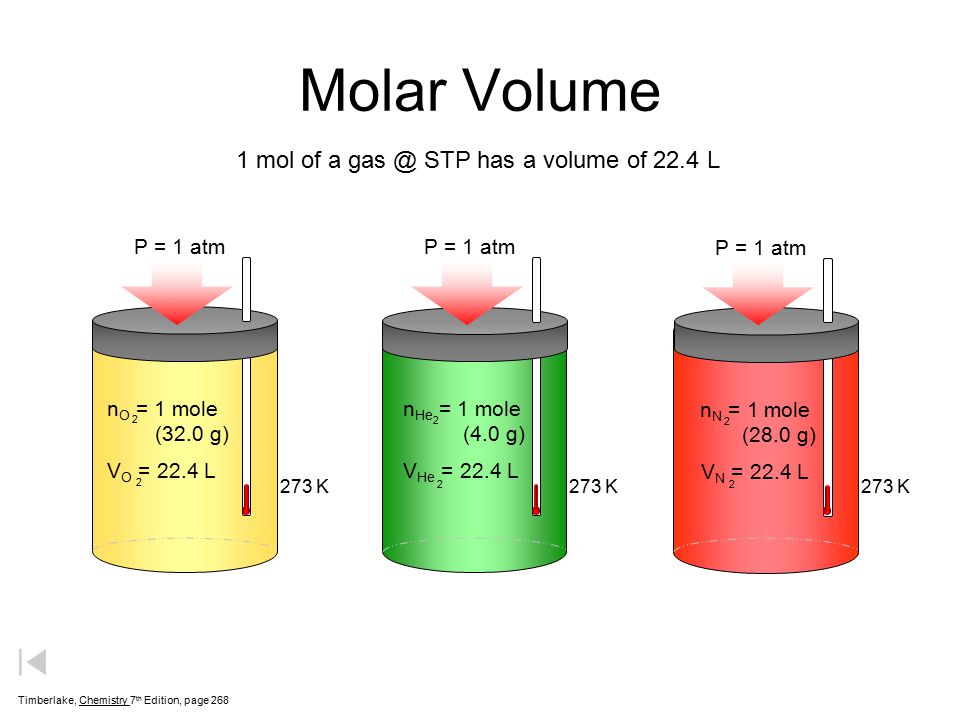
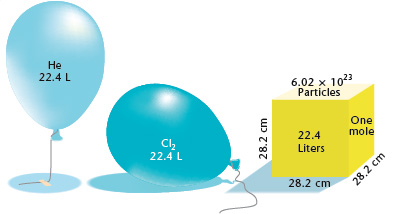
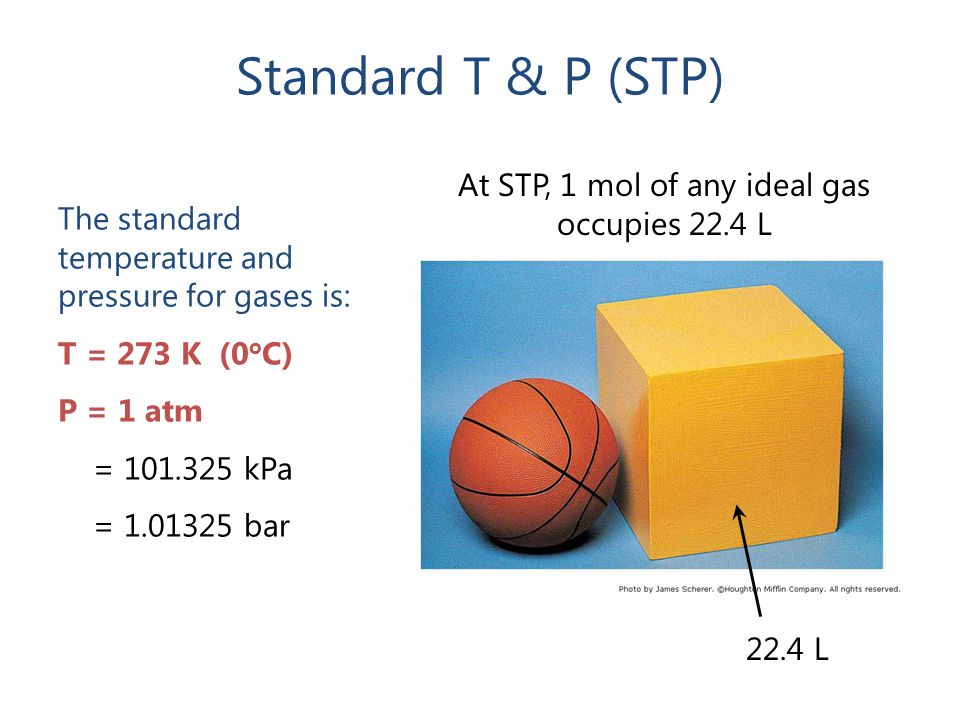
Gases & Stoichiometry. Molar Volume 1 mol of gas = 22.4 L molar volume What volume would be occupied by 0.77 moles of helium gas at STP? - ppt download
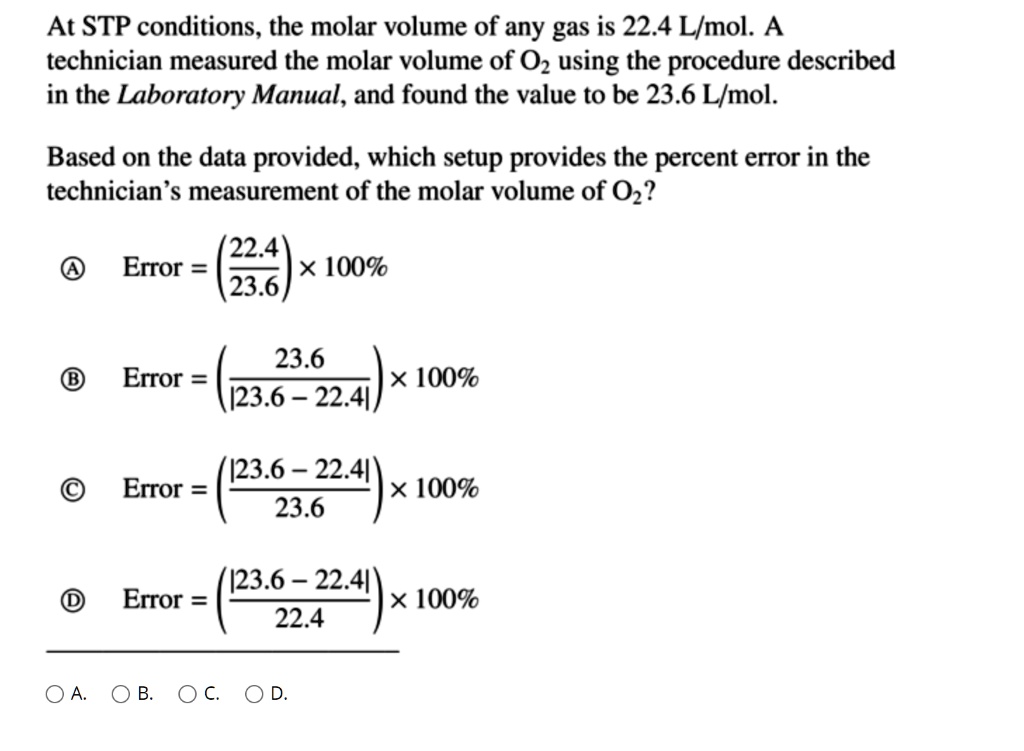
SOLVED: At STP conditions, the molar volume of any gas is 22.4 L/mol. A technician measured the molar volume of 02 using the procedure described in the Laboratory Manual, and found the

The volume occupied by a mole of gas (molar volume,V_(m)) at STP is called the standard molar vo... - YouTube

One mole of an ideal gas at standard temperature and pressure occupies 22.4 L(molar volume). What is the ratio of molar volume to the atomic volume of a mole of hydrogen ? (